Overview
Hepacure Pharma is an early-stage company focused on a novel peptide therapy for the treatment of fatty liver diseases. Fatty liver diseases or non-alcoholic fatty liver disease (NAFLD) refers to a range of conditions caused by a build-up of fat in the liver. High levels of fat in the liver are also associated with an increased risk of serious health conditions, such as diabetes, high blood pressure and kidney disease.
The unmet need
NAFLD is a condition in which fat accumulates in the liver. Nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) are types of NAFLD.
The disease starts with inflammation in the liver, followed by fibrosis (type of scar tissue), making these cells nonfunctional. The disease can progress to carcinoma, which is a non-curable cancer. The mortality rate in advanced stages is expected to reach 40% by 2030.
Today, the pipeline in fatty liver disease market is limited. On March 2024, the FDA approved the first medication for NASH (Resmetirom, oral route) and there are a few clinical trials in different phases carried out for this indication.
The Market
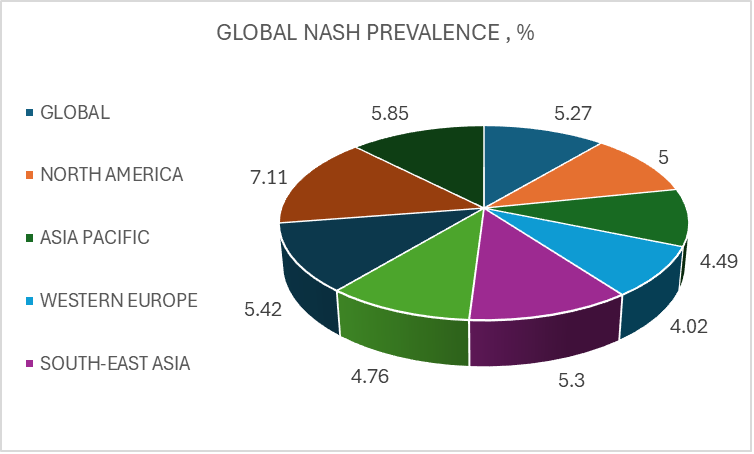
NAFLD is a major cause of liver disease worldwide. The estimated global incidences of NAFLD is 47 cases per 1,000 population, leading to market size of 100 million patients in 2030.
Projections in the prevalence of NAFLD (Clin Mol Hepatol. 2023;29(Suppl): S32-S42. doi : https://doi.org/10.3350/cmh.2022.0365)
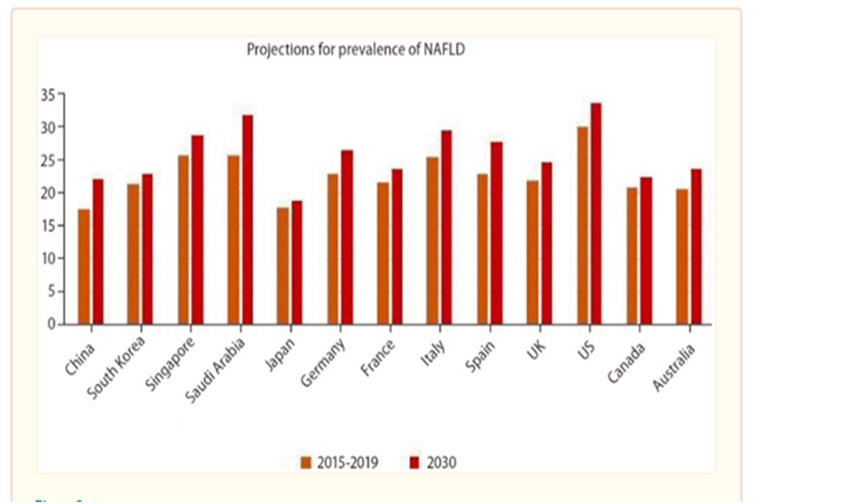
The global prevalence of NAFLD is forecasted to reach 25%-30% in 2030 and 55.4% by 2040
Economic Burden of fatty liver disease
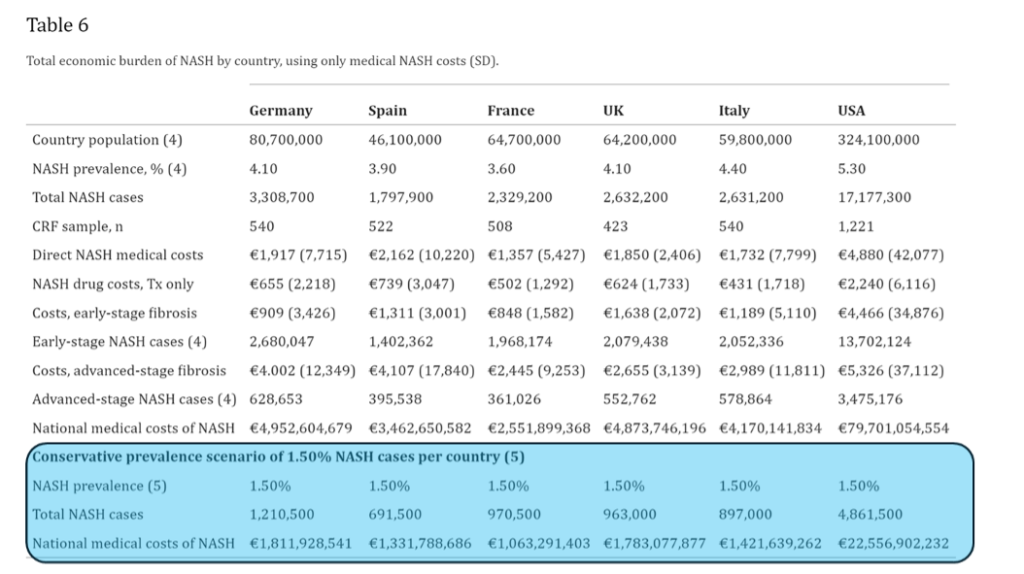
In 2020, the GAIN study: Cost of non-alcoholic steatohepatitis in Europe and the USA (DOI: 10.1016/j.jhepr.2020.100142) provided real-world data on the direct medical, non-medical, and indirect costs associated with NASH, including patient-reported outcomes in Europe and the USA, showing a substantial burden on health services and individuals.
The GAIN study summarizes the economic burden in different countries using only medical NASH cost:
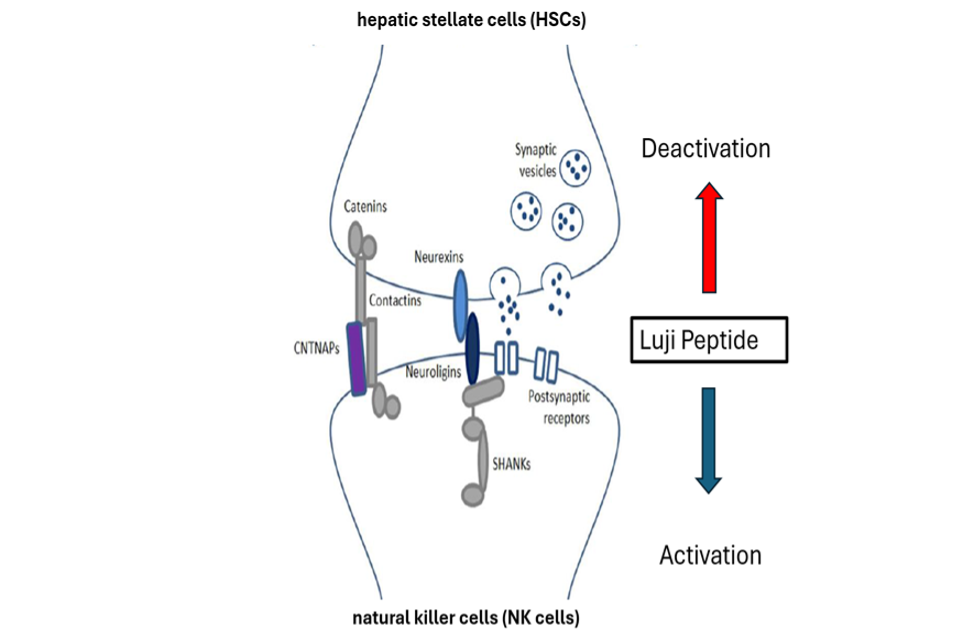
intraperitoneal administration of the Luji peptide in vivo study in a fibrosis mouse model (carbon tetra chloride induced model), delays the fibrosis process and improves the histologic damage of the liver by reducing the inflammation, the fibrosis and reducing damage of liver enzymes.
The modulator Luji peptide is used in a method of treating, attenuating and/or preventing progression of liver disorders including Steatotic Liver Disease.
The Luji peptide is targeted to be administered as once-weekly injection for early stage of the disease (F2 stage of Fibrosis)
Team
Klang Shmuel, CEO
Expert in health technology assessment, with over 25 years of senior management experience in the health system, health economics and the drug basket, including heading Pharmacy and Pharmacology at Clalit Health Services- (Israel’s largest Health service organization)
Lecturer in University of Haifa, School of Public Health in health management.
Ph.D degree from the School of Pharmacy/Faculty of Medicine at the Hebrew University.
Master’s degree in health systems management from Ben Gurion University in the Negev.
MBA – Executive program of Business Management – TLV University, Leon Recanati Graduate School
Prof. Safadi Rifaat, Founder.
Prof. Safadi is a full Professor in Medicine, Gastroenterology and Hepatology, Faculty of Medicine, Hadassah University Hospital, Jerusalem & a visiting Scholar, at the division of Liver Diseases, Mount Sinai School of Medicine in NY.
Prof. Safadi is the current treasurer of the Israeli Association of Gastroenterolgy and Liver (since 2019), past Chairman of The Israel Association for the Study of the Liver (2014-2016). Prof. Safadi is a member of several international Liver associations committees such as EASL (scientific committee and governing board), AASLD (International Relations Advisory Committee). He is the Director of Liver Unit, Institute of Gastroenterology and Liver Diseases, Division of Medicine, Hadassah. Prof. Safadi is the Director of Liver Unit & Clinical Research Center, Holy Family Hospital, Nazareth, Israel. Prof. Safadi had his residency in Internal Medicine & Gastroenterology at Hadassah University Hospital, Jerusalem. His clinical focus is in viral hepatitis, Cirrhosis complications and liver cancer, immune diseases of the liver and liver transplantation. With his laboratory team, the interplay research between lymphocyte subsets and hepatic fibrogenesis uncovered novel pathways and challenges. He is interesting in novel metabolic checkpoints of Natural Killer Cells (NK cells). He received many awardees and research grants and published more than 170 manuscripts in high rated professional journals within the clinical and basic research liver fields. He is a reviewer and a member for editorial boards of several academic journals.
Contact info:
Dr. Shmuel Klang – CEO
shmuel@hepacure-pharma.com
+972- 50-626 3089